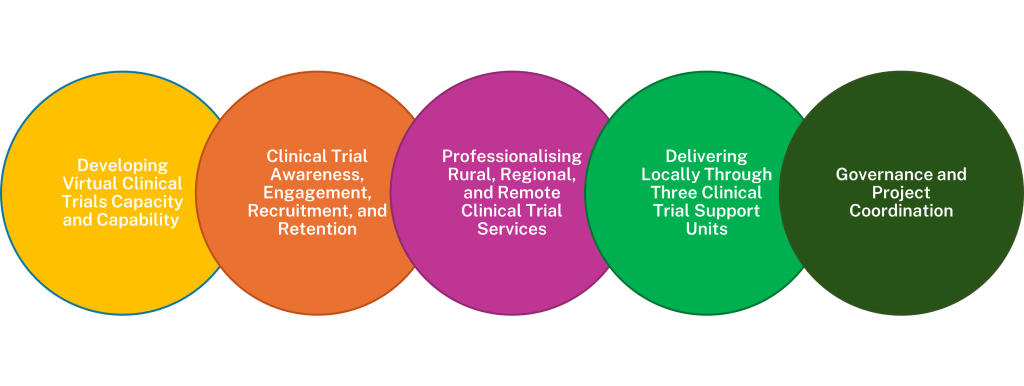
The Rural, Regional and Remote Clinical Trial Enabling Program, is
- supported by $30.6M funding from the Australian Government Medical Research Future Fund
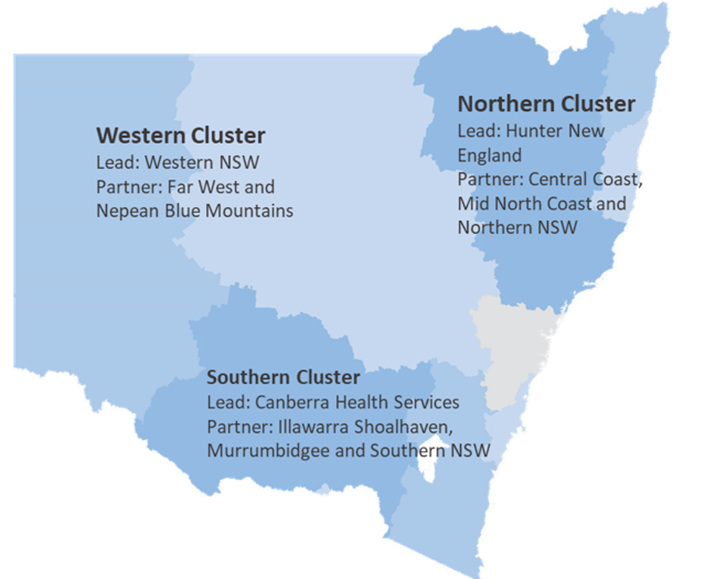
- being delivered by NSW Health in partnership with ACT Health and state and national partners
- establishing increased and more equitable access to clinical trials for people in rural, regional, and remote NSW and the ACT.
- locally led by three Clusters (Northern, Western and Southern) in partnership with state and national partners, and
- centrally supported by the Office for Health and Medical Research, NSW Health
| The Program will deliver a clinical trial ecosystem which meets the needs of local communities, attracts sponsors, and embeds a sustainable clinical trials model for R3 NSW and ACT. |
Subscribe to the Rural, Regional and Remote Clinical Trial Enabling Program newsletter

MRFF ID: MRFRR000047
Updated 1 day ago