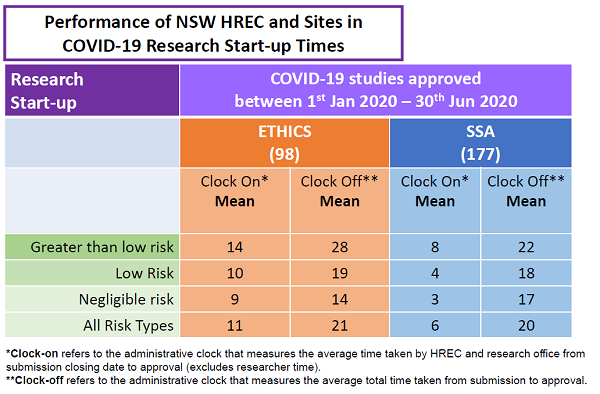
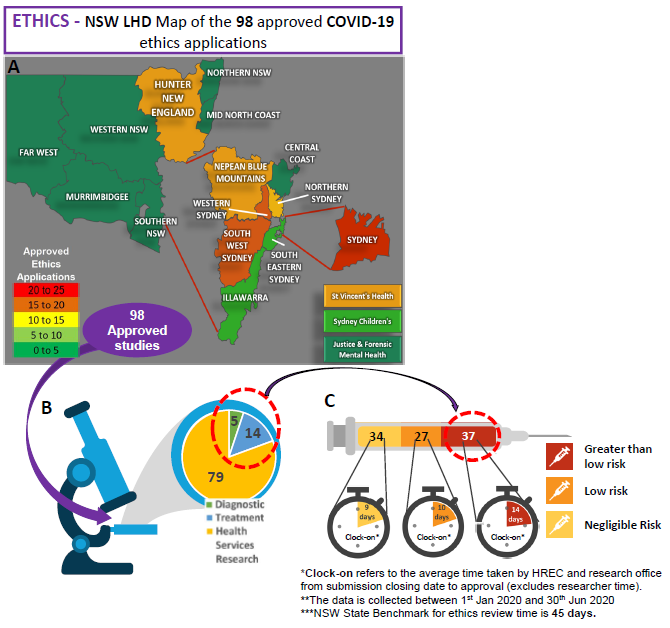
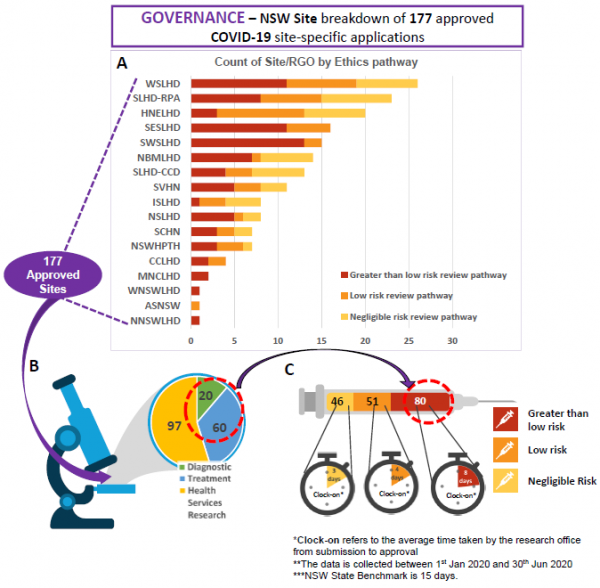
Research Ethics and Governance metrics
Improving research ethics and governance approval timelines, particularly for clinical trials, is a key priority for NSW.
The Office for Health and Medical Research collects data from NSW local health districts, specialty health networks and NSW Ambulance to generate ethics and governance metrics for health and medical research, including clinical trials.
Why we collect Ethics and Governance metrics
The ethics and governance metrics collected by the Office for Health and Medical Research provide quantitative evidence and enable an accurate portrayal of approval timelines for health and medical research projects in NSW.
Work is currently being undertaken to collect quarterly data for start-up times and report on all the performance of all Research Offices. Analysis of these dataset will identify start-up time improvement opportunities across the state.
This Metrics Manual document outlines Key Performance Indicator (KPI) thresholds and illustrations of measurement calculations.
| Metrics Manual | |
Metrics Activity Reports
Financial Year 2025:
| Q1 2025 | |
Financial Year 2024:
| Q1 2024 | |
| Q2 2024 | |
| Q3 2024 | |
| Q4 2024 | |
Updated 3 months ago