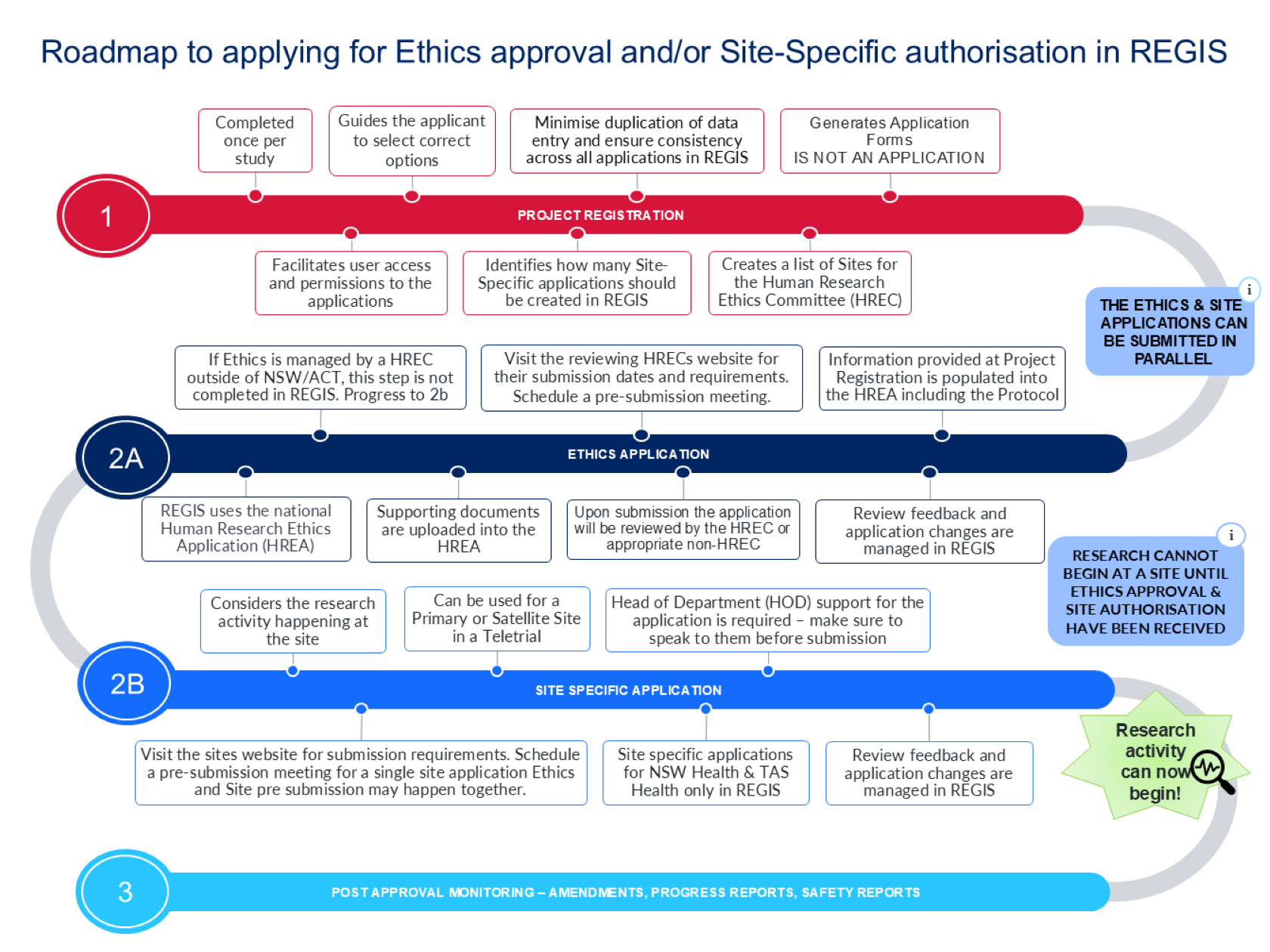
Introducing the roadmap to applying for Ethics approval and/or Site-Specific Authorisation in REGIS
Updates have been made to the Project Registration form based on user feedback and involve refinements to the text and images, aimed at enhancing clarity and improving the overall user experience. The changes will make it easier for users to understand the project registration process, including the requirements for completing the form, study team permissions and delegations, and the steps that follow submission. The roadmap will appear at the beginning of and the end of each application form.
NSW Health Research Handbook
The OHMR REGU team is excited to announce the release of the updated NSW Health Research Handbook 1.1 and an initial set of Standard Operating Procedures (SOPs). These resources are critical tools to guide safe, ethical, and high-quality human research across NSW Health organisations. Designed to be a living document, these resources outline the essential requirements for conducting research, while allowing local health districts/organisations to adapt highlighted sections (in yellow) to fit their specific contexts.
This release also marks the beginning of our second 6-month consultation period to refine these resources and further strengthen NSW Health’s commitment of embedding a research-enabling culture. Your insights are crucial to ensuring the Handbook and SOPs are practical, user-friendly, and aligned with the needs of researchers, Research Development Offices, Research Governance Officers, and Human Research Ethics Committees. We encourage you to share these resources widely with your teams and networks and use them extensively.
To contribute your insights, please submit feedback using the Feedback Form by 1 July 2025. For lengthy feedback or concerns, email MOH-ResearchEthics@health.nsw.gov.au.
Access the Summary of Changes to the NSW Health Research Handbook 1.1
Access the NSW Health Research Handbook 1.1
Access the NSW Health Roles & SSA Responsibilities and Access Request Decision Tree
Watch the NSW Health Research Handbook webinar
Updated 1 week ago