Clinical Trial Monitoring & Safety
NSW Health’s Safety Monitoring and Reporting for Clinical Trials Conducted in NSW public health organisations policy directive applies to all clinical trials conducted within public health organisations in NSW.
The policy, released on 27 October 2017, outlines the safety monitoring and reporting requirements for trials involving investigational medicinal products or investigational medical devices (referred to as therapeutic good trials) and also the safety monitoring and reporting requirements for trials involving other types of intervention (referred to as non-therapeutic goods trials).
Safety notifications and reporting pathways for therapeutic goods trials
Safety notifications for therapeutic goods trials
A summary table of safety notifications to the Human Research Ethics Committee and Research Governance Officers
View safety notification summary table
- Type of event
- Who reports
- To whom
- When
- How
- Significant Safety Issue (SSI) implemented as an Urgent Safety Measure (USM)
- Sponsor/Delegate
- The reviewing HREC (and all investigators participating in the study)
- As soon as possible and no later than 72 hours of the sponsor becoming aware of the USM
- SSI Notification Form; Sponsor’s template
- Significant Safety Issue (SSI) not implemented as an Urgent Safety Measure (USM)
- Sponsor/Delegate
- The reviewing HREC (and all investigators participating in the study)
- Within 15 days of the sponsor becoming aware of the SSI
- SSI Notification Form; Sponsor’s template
- All Significant Safety Issues (SSIs)
- Principal Investigator
- The RGO for the site where the event occurred
- As soon as possible and no later than 72 hours of the PI becoming aware of the SSI
- SSI Notification Form; Sponsor’s template
- Suspected Unexpected Serious Adverse Events (SUSARs) and Unanticipated Serious Adverse Device Effects (USADEs) occurring at the site
- Principal Investigator
- The RGO for the site where the event occurred
- Within 72 hours of the PI becoming aware of the event
- SUSAR/USADE/URSAE Notification Form
- Investigator’s Brochure Updates/Addenda
- Sponsor/Delegate
- The reviewing HREC
- As and when updates are generated
- Submitted with a cover sheet or as part of an annual progress/annual safety report
- Annual Safety Report
- Coordinating Principal Investigator or Sponsor/Delegate
- The reviewing HREC
- Within annual progress report sent to the HREC or aligned with the safety reporting cycles of global companies
- Annual Progress Report or sponsor’s template
Reporting pathways for therapeutic goods trials
As illustrated below, sponsors may report directly to NSW Human Research Ethics Committee; however, they must ensure that all communications sent to the Human Research Ethics Committee adequately identify the trial and provide context in relation to the Human Research Ethics Committee’s role (e.g. whether there is any impact on patient safety, trial conduct or trial documentation).
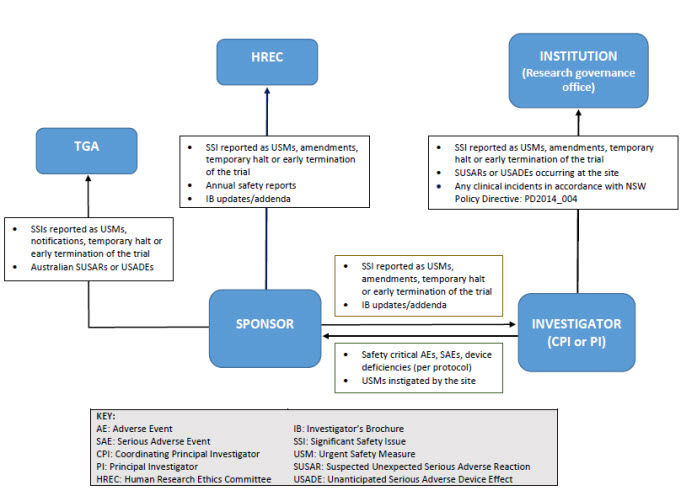
Safety notifications and reporting pathways for non-therapeutic goods trials
Safety notifications for non-therapeutic goods trials
View safety notification summary table
- Type of event
- Who reports
- To whom
- When
- How
- Significant Safety Issue (SSI) implemented as an Urgent Safety Measure (USM)
- Sponsor/Delegate
- The reviewing HREC (and all investigators participating in the study)
- As soon as possible and no later than 72 hours of the sponsor becoming aware of the USM
- SSI Notification Form; Sponsor’s template
- Significant Safety Issue (SSI) not implemented as an Urgent Safety Measure (USM)
- Sponsor/Delegate
- The reviewing HREC (and all investigators participating in the study)
- Within 15 days of the sponsor becoming aware of the SSI
- SSI Notification Form; Sponsor’s template
- All Significant Safety Issues (SSIs)
- Principal Investigator
- The RGO for the site where the event occurred
- As soon as possible and no later than 72 hours of the PI becoming aware of the SSI
- SSI Notification Form; Sponsor’s template
- Unexpected & Related Serious Adverse Event (URSAEs) occurring at the site
- Principal Investigator
- The RGO for the site where the event occurred
- Within 72 hours of the PI becoming aware of the event
- SUSAR/USADE/URSAE Notification Form
- Annual Safety Report
- Coordinating Principal Investigator or Sponsor/Delegate
- The reviewing HREC
- Annually (within the annual progress report)
- Annual Progress Report
Reporting pathway for non-therapeutic goods trials
As illustrated below, sponsors may report directly to NSW Human Research Ethics Committees; however, they must ensure that all communications sent to the Committee adequately identify the trial and provide context in relation to the Committee’s role (e.g. whether there is any impact on patient safety, trial conduct or trial documentation).
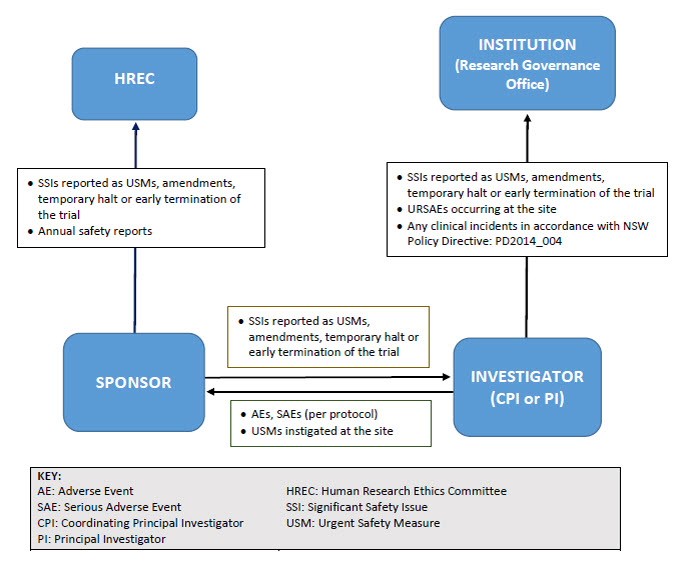
Resources and forms
Resources
-
Sample letter to Sponsor: Unwanted safety reports
DOCX - 28 KB
Forms
-
Significant Safety Issue Notification Form
DOCX - 51 KB
-
Local SUSAR/USADE/URSAE Notification Form
DOCX - 56 KB
Definitions
Therapeutic Goods Trials: Trials investigating the safety and/or effectiveness of medicines, biologicals or medical devices.
Non-Therapeutic Goods Trials: Trials other than a Therapeutic Goods Trial (e.g. radiotherapy, surgery, psychotherapy trials).
Suspected Unexpected Serious Adverse Reaction (SUSAR): An adverse reaction that is both serious and unexpected.
Unanticipated Serious Adverse Device Effects (USADEs): A serious adverse device effect which by its nature, incidence, severity or outcome has not been identified in the current version of the risk analysis report (and/or Investigator’s Brochure/Instructions for Use).
Urgent Safety Measure (USM): A measure required to be taken in order to eliminate an immediate hazard to a participant’s health or safety.
Significant Safety Issue (SSI): A safety issue that could adversely affect the safety of participants or materially impact on the continued ethical acceptability or conduct of the trial.
Unexpected & Related SAEs (URSAE): An adverse event that is:
- Serious – meets the definition of a serious adverse event
- Related – resulted from administration of the trial intervention
- Unexpected – the event is not described in the protocol as an expected occurrence.
Frequently asked questions
Will the new reporting requirements be implemented for trials with existing ethical approval?
Yes. The transition should be made as simple as possible and a Sample letter to Sponsors has been produced stating that the requirements apply to all new and existing trials (including trials opened before November 2016). The letter provides ‘blanket approval’ for the change so that individual protocol amendments to NSW Human Research Ethics Committees/Research Governance Officers will not be necessary.
The National Health and Medical Research Council’s Guidance only applies to therapeutic goods. How should other trials be handled?
NSW Health’s Policy Directive provides a framework for safety monitoring and reporting for non-therapeutic goods trials (e.g. surgery or psychotherapy) in order to standardise the requirements for all clinical trials. The same reporting principles and requirements that are described in the National Health and Medical Research Council’s Guidance have been applied to non-therapeutic goods trials.
Why has the Policy Directive used new terminology for non-therapeutic goods trials?
The terms ‘adverse reaction’ and ‘suspected unexpected serious adverse reaction’ (SUSAR) are not appropriate for device trials (as no reaction is taking place), so they are also not appropriate for non-therapeutic goods trials. The SUSAR-equivalent for a non-therapeutic good trial would be an Unexpected and Related Serious Adverse Event (URSAE).
What changes to reporting requirements have been made?
Human Research Ethics Committees will no longer receive single case AEs, SAE/SARs and SUSARs* or device/non-therapeutic good trial equivalents or six monthly line listings.
Human Research Ethics Committees will receive all significant safety issues, annual safety reports and investigator’s brochure updates.
Research Governance Offices will no longer receive single case AEs, SAE/SARs and external SUSARs* or device/non-therapeutic good equivalents or six monthly line listings.
Research Governance Offices will receive all significant safety issues (SSIs), any local SUSARs/USADEs/URSAEs and any research-related events that meet the definition of an incident (PD2014_004).
*Note: If a SAE/SAR/ SUSAR meets the definition of an SSI, it will be reported to the HREC/RGO through that reporting mechanism.
How will sponsors and investigators be informed of these changes?
NSW Health will confirm the changes to industry through Medicines Australia and the MTAA.
NSW Research Offices should communicate the changes as widely as possible to their own research active departments and any stakeholders they have contact with. NSW Research Offices may also direct stakeholders to NSW Health’s clinical trials webpage.
If the public health organisation is the sponsor, how should safety reporting be managed?
This is a business decision of the organisation. It is likely, as is current practice, that for clinical trials, many sponsor functions are delegated to the CPI or a coordinating centre. It is recommended that PHO-sponsors develop written procedures for their ‘sponsored’ trials so that all parties are aware of their responsibilities.
Sponsors should maintain oversight of functions that they delegate to ensure that they are being handled appropriately. This usually consists of some form of audit and monitoring activities to detect and rectify poor compliance.
Note: The NHMRC Guideline has not changed the role of the non-commercial trial sponsor. Instead, it has highlighted the requirements outlined in international Good Clinical Practice Guidelines adopted by the Therapeutic Goods Administration.
Will the use of standard safety reporting forms be mandated?
The use of two forms is mandated:
- Significant Safety Issue (SSI) Notification Form (reporting from sponsor to HREC)
- SUSAR/USADE/URSAE Notification Form (reporting from local investigator to their local RGO)
It is likely that these forms will evolve following validation (user acceptance testing). Please encourage users to provide real-time feedback on the utility of these forms (and any suggested improvements) so that relevant information can be provided for future versions. NSW Health will monitor the need to develop other standard forms but at present, sponsors may provide annual safety reports or updated IBs with a cover sheet.
Does every amendment relating to adverse events meet the definition of significant safety issue?
No, SSIs are safety issues that adversely affect the safety of participants or materially impact on the continued ethical acceptability or conduct of the trial. Normally, only issues that alter the risk benefit balance of the clinical trial meet the definition of a SSI.
An Urgent Safety Measure is defined as a measure required to be taken in order to eliminate an immediate hazard to the participant's health or safety. Do all urgent interventions that occur in a clinical trial meet the definition of an Urgent Safety Measure?
No. An urgent safety measure is an event that requires a change to trial procedures or the addition of unapproved trial procedures which are not defined in the protocol. Therefore, where urgent interventions are managed in accordance with the protocol, they are not considered USMs. For example an investigator may implement an immediate IMP dose reduction, in response to an observed toxicity, in accordance with the protocol’s dose modification rules.
How can HRECs monitor the welfare of the affected participant if they no longer get individual case reports such as SAEs and SUSARs?
In a single ethical review system as a trial progresses, the HREC’s responsibility is to keep under review the overall risk benefit balance of the trial, not the welfare of individual participants. The duty of care for individual participants lies with the Principal Investigator and their institution. Sponsors (and not HRECs) are responsible for monitoring that investigators are adequately executing their responsibilities in relation to participant safety.
Why was direct reporting (sponsor to Human Research Ethics Committee) introduced and should all NSW Human Research Ethics Committees accept direct reporting from the sponsor?
Yes. All NSW Human Research Ethics Committees and public health organisations should accept direct reporting in accordance with the National Health and Medical Research Council’s Guidance. The rationale for direct reporting is as follows:
Reduced administrative burden
Sponsors are finding it increasingly difficult to recruit CPIs due to the high administrative burden of the role. Much of that burden stems from the requirement for the lead site to transmit information generated by the sponsor to the Human Research Ethics Committee and also to other PIs.
By allowing the sponsor to send the information directly to the Human Research Ethics Committee, providing a copy in parallel to the CPI (and PIs where relevant), a two-step process becomes a single step process and the burden on the CPI is reduced.
In addition, allowing the sponsor to report directly reduces their monitoring burden as they no longer have to check (monitor) that the lead site has actually sent the report to the Human Research Ethics Committee.
Reporting timelines
The National Health and Medical Research Council’s Guidance requires sponsors to submit SSI reports within 72 hours (USMs) or 15 days (for other SSIs). Reporting via the CPI will make these timeframes harder to meet in some trials.
Alignment with national and international practice
Direct reporting is logical in countries operating a single ethical review model for multicentre trials (e.g. National Mutual Acceptance). Both the FDA and EU accept direct reporting and other states in Australia have already transitioned to direct reporting.
Note: Sponsors may delegate the task of ‘safety reporting to the HREC’ to the CPI or another third party. If sponsors choose to report directly, CPIs and PIs should be provided with any report/ information sent to the HREC that impacts on their role.
What advice should be provided to sponsors in relation to direct reporting to the Human Research Ethics Committee?
Sponsors should be made aware of two key requirements when reporting directly to NSW Human Research Ethics Committees:
- any direct reporting should take into account Section 5.2.20 of the National Statement. If sponsors limit their communication to the provision of reports, these communications will not influence (or be perceived to influence) the ethical review process
- sponsors must ensure that all reports/documents sent to the Human Research Ethics Committee (where standard templates are not available) adequately identify the trial and provide some context in relation to the Human Research Ethics Committee’s role; such as the impact on patient safety, trial conduct and/or trial documentation*
- sponsors must ensure that all reports/documents sent to the HREC (where standard templates are not available) adequately identify the trial and provide some context in relation to the HREC’s role; such as the impact on patient safety, trial conduct and/or trial documentation*.
*Note: Any changes to trial documents (e.g. protocols, PICFs, updated IBs) should be provided to the HREC using the standard amendment reporting process.
Can sponsors send HRECs an Investigator’s Brochure (IB) as an Annual Safety Report for therapeutic goods trials?
Yes. For therapeutic good trials, the National Health and Medical Research Council’s Guidance provides advice on the content of the annual safety report but allows flexibility; both in terms of the timing of that report and also the format.
For example, an annual safety report may consist of a cover sheet that cross-references the information contained within an IB/DSUR/DSMB report, which should be provided as an attachment. Annual safety reports must also:
- adequately identify the trial and provide some context in relation to the Human Research Ethics Committee’s role
- confirm that the safety monitoring plans described in the protocol/Human Research Ethics Committee application are being followed.
How should the Annual Safety Report for Non-Therapeutic Goods trials be provided to the HREC?
For non-therapeutic goods trials, the requirement for an annual safety report will be fulfilled by providing a summary of the trial’s evolving safety profile in the Annual Progress Report sent to the Human Research Ethics Committee.
What should HRECs do if they receive reports that are no longer required by the NHMRC Guidance?
Confirm receipt and inform the reporter that the event(s) sent are no longer required by NHMRC Guidance or NSW policy. These reports do not have to be reviewed by the committee.
Do investigators still need to print/review/file reports that are not required to be sent to sites by the National Health and Medical Research Council’s guidance?
No. The National Health and Medical Research Council’s guidance has aligned with EU Clinical Trial Regulation 536 which has removed the requirement for sponsors to send reports such as individual case SUSARs and six monthly line listings to investigators. However, sponsors may be obliged to follow global company policies that mandate the reporting of SUSARs/line listings. In these instances, it is good practice for investigator sites to confirm receipt of these reports and sites should follow sponsor guidance on how this may be achieved. The National Health and Medical Research Council’s guidance does not; however, oblige investigators to print, review and file ‘routine’ reports that have no bearing on participant safety or trial conduct.
Should the TGA receive all significant safety issues, even those that are solely due to trial conduct (and not the IMP/IMD)?
Yes. The TGA wishes to be notified of all events that are relevant to participant safety and therefore require notification of all SSIs, including those that have resulted from serious adverse events that are associated with a trial procedures. It should be noted however that the TGA should receive notification that a SSI has occurred but any resulting amendment (e.g. revising trial documentation) should be submitted to the HREC only.
What happens if, having read the Policy and these frequently asked questions, we have additional questions?
Please e-mail any queries to researchethics@doh.health.nsw.gov.au.
Updated 4 years ago