Surgeons perform 60,000-odd knee replacements in Australia per year, with each costing around $30,000. But about one in five patients, roughly 12,000 people, are unhappy with the outcome.
That’s a significant health problem in terms of pain and restricted movement. It’s also a poor return on investment of hundreds of millions of dollars.
But a small Sydney company is hoping to make a serious dent in that level of dissatisfaction by using advanced technology to simulate each operation many times over so they can plan and implement the best possible intervention and rehabilitation program.
“We aim to reduce it to below one in 20 people,” says entrepreneur Bede O’Connor, co-founder of 360 Knee Systems Pty Ltd.
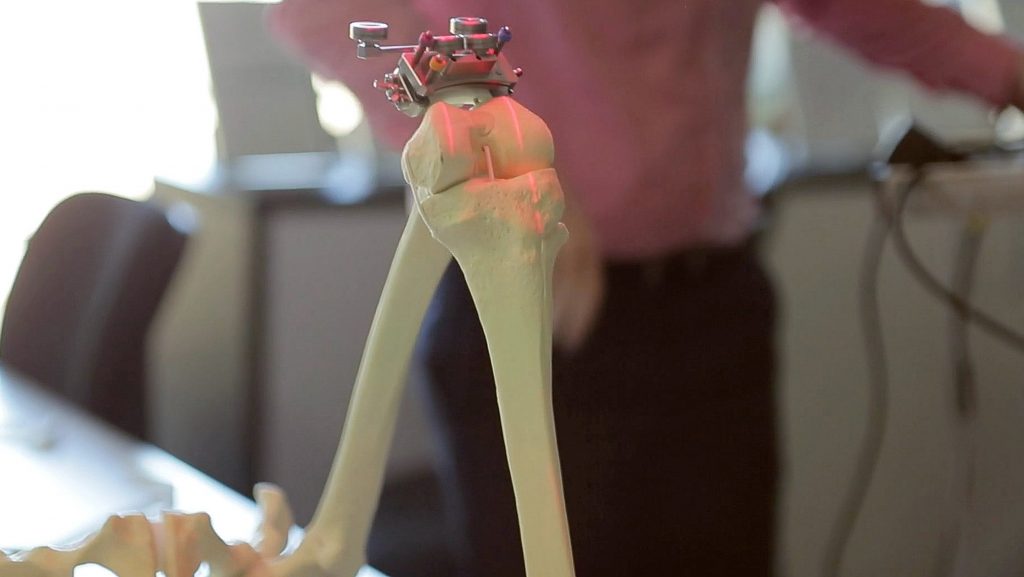
With assistance from the NSW Medical Devices Fund, 360 Knee Systems is launching into Japan, China, and India this year, partnered with multinational healthcare giant, Johnson & Johnson.
It’s also beginning operations in France and expanding its existing business in the US, where about one million knee replacements are carried out each year. Not bad for a company that was founded just five years ago in a hugely competitive field by O’Connor and biomedical engineer, Brad Miles. It now employs about 35 engineers and 15 physiotherapists.
“These days, the major differences in successful replacement outcomes come from adjunct technologies – the collection of data, the simulation of the operation, and planning and moving a patient through the system,” says O’Connor. “We believe we’re the best in the world at that.”
In 2014, the Medical Devices Fund provided $2 million to help the fledgling company get off the ground, based on patented technology to simulate and test outcomes of knee operations in a range of circumstances.
That money helped pay for 360 Knee Systems’ successful submission for approval by the US Food and Drug Administration (FDA) – a critical condition for entry to US and global medical device markets.
Insights from virtual reality
A typical 360 Knee Systems patient is 55 to 75 years old and is suffering pain and restricted movement due to osteoarthritis. The process starts once they are referred to an orthopaedic surgeon.
Even before that appointment, the patient will meet with a 360 Knee Systems physiotherapist, who begins a six-week assessment of their ability to perform prescribed exercises and the level of pain this incurs. The patient reports via a Fitbit-style device and face-to-face using an enabled iPad.
If the patient and surgeon decide to go ahead with a knee replacement, this information is used in conjunction with a CT scan to inform the simulation software.
This allows a virtual operation to be performed 30 or 40 times over to come up with the best solution in terms of factors such as which implant to use, and how it’s aligned. This allows for meticulous operation planning, the details of which can be fed into computer navigation and robot systems to assist the surgeon.
Finally, 360 Knee Systems provides a complete rehabilitation regime and oversees it for up to a year.
This helps to ensure that the replacement process happens smoothly, and consolidates what would typically be several separate specialist stages – assessment, imaging, surgical intervention, and physiotherapy.
A state for entrepreneurs
Last year, 360 Knee Systems received another NSW Medical Devices Fund grant of $2.5 million to develop technology that will use advanced manufacturing techniques to customise existing implant products to individual patients.
“We’re hoping to do this within two years,” says O’Connor. “In medical innovation, speed matters.”
360 Knee Systems is able to sell its services in two ways: as adjunct technologies distributed by large implant manufacturers such as Johnson & Johnson to make their products more attractive; and as a service to private health insurers to help reduce the overall costs of knee replacements.
As O’Connor explains, assistance from the Medical Devices Fund goes well beyond grants. It’s provided them with access to the resources of the NSW healthcare system, plus crucial insights into the thinking and planning that’s going on in the medical devices arena.
“NSW Health is really entrepreneurial, and is looking to create businesses in NSW,” says O’Connor.
“Medical technology is a big employer of STEM graduates, and there’s now heaps of evidence all around the world that wherever medical R&D and clinical studies are done, there are always better medical outcomes for patients.”
By Tim Thwaites
Updated 4 years ago
