Every year, heart attacks send around 57,000 Australians to hospital and, in 2018 alone, they took more than 7300 lives – an average of 20 each day. Those who survive run a higher risk of developing heart failure.
Associate Professor James Chong, a cardiologist at Sydney’s Westmead Hospital, witnesses their pain firsthand. He also leads the Westmead Institute Cardiac Regeneration Group, where his team is harnessing the therapeutic potential of stem cells and endogenous heart cells in the hope that, one day, they’ll help strengthen patients’ hearts.
Using a protein that stimulates tissue growth, Chong and colleagues modified heart scar tissue formation in pigs after a heart attack. Treated porcine subjects also had better heart function and were less likely to die than their untreated counterparts.
“That’s what excited me about our findings,” Chong says. “We saw increased survival, and I think we’re changing scar tissue with this therapy in a way that, to my knowledge, isn’t happening in current treatments.”
After ticking off a few more experiments, he hopes the work, which was unveiled in Science Translational Medicine in January, will enter human clinical trials.
Change of heart
Coaxing an adult heart into repairing itself is a relatively new idea because, until recently, it was considered impossible.
Most of a healthy heart’s fist-sized volume comprises smooth muscle cells called cardiomyocytes. Under the coordinated control of pacemaker cells, the heart muscle contracts then relaxes to produce the familiar “lub-dub” beat as blood shuttles around the body.
During a heart attack – also known as myocardial infarction – blood supply to the heart is blocked. Cardiomyocytes usually nourished by that blood start dying.
Throughout the 20th century, many doctors thought that was the end of the story – once dead, cardiomyocytes could not grow back. But recent studies have shown that healthy human cardiomyocytes slowly regenerate during our lives. The heart harbours cardiac-specific stem cells, and differentiated heart cells can re-enter the cell cycle.
These findings “captivated” Chong: “I was always taught in medical school that the heart couldn’t regenerate itself, and we now know from some very good studies that’s not entirely true.”
Now Chong – who received from NSW Health an Early-Mid Career Fellowship in 2018 and a Cardiovascular Clinician Scientist Grant in 2019 – is working with what are known as “induced pluripotent stem cells” to help shore up injured heart tissue.
Unlike tissue-specific stem cells, induced pluripotent stem cells are “reprogrammed” adult cells that can give rise to any cell. In theory, a patient’s skin cells could be cultured into healthy cardiomyocytes and engrafted onto their damaged heart.
Chong’s team is developing protocols to turn out millions or billions of cells at a time using devices called bioreactors.
“The big advantage stem-cell-derived cardiomyocytes have over a heart transplant is that there is a potentially indefinite source of cardiomyocytes – important because there’s a real shortage of donor hearts,” he says.
A scar is born
When cardiomyocytes die, another cell population – fibroblasts – springs into action, replacing damaged tissue with a scar made of collagen. But scar tissue increases a patient’s odds of developing arrhythmia, where the heart beats irregularly, too slowly or too quickly.
For this reason, scar tissue “has always been considered the bad guy in heart disease,” Chong says. “In part, we think it prevents normal heart function, which is a coordinated movement.”
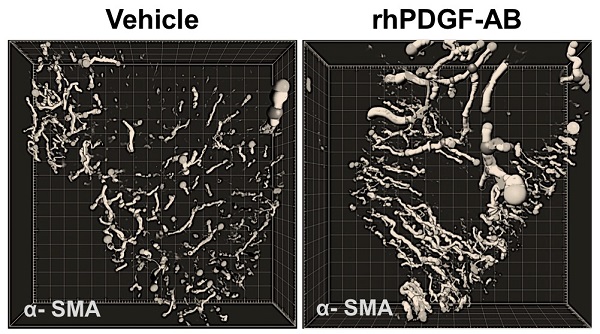
Building on previous experiments on rodent heart scar tissue, Chong and his crew administered a growth-stimulating protein called platelet-derived growth factor, or PDGF, to a pig model of heart attack.
They found PDGF-treated pigs were less likely to die or develop arrhythmias over four weeks following treatment than the untreated pigs, despite there being no difference in the amount of heart scar tissue between groups.
“That led us to think maybe it’s not the quantity of scar, but the quality of scar,” Chong says.
Microscope imagery showed collagen fibres in the treated pigs’ scars were more aligned and uniform than those in the control group.
They also saw more blood vessel growth in treated pigs’ hearts.
So what happened? His group is still working on the answer, but Chong suspects PDGF influences different cell types within the injured heart, including endothelial cells, which line blood vessels, and fibroblasts.
Nailing exactly how PDGF works will also help researchers gauge an ideal therapeutic window. Similar or better results might be gained by tweaking when treatment starts or how long it lasts, Chong says: “In this case, it was 7 days of treatment, but we might even be able to get away with less.”
By Bel Smith
Updated 4 years ago
